Lung Cancer Data
Market Dynamics and Outcomes
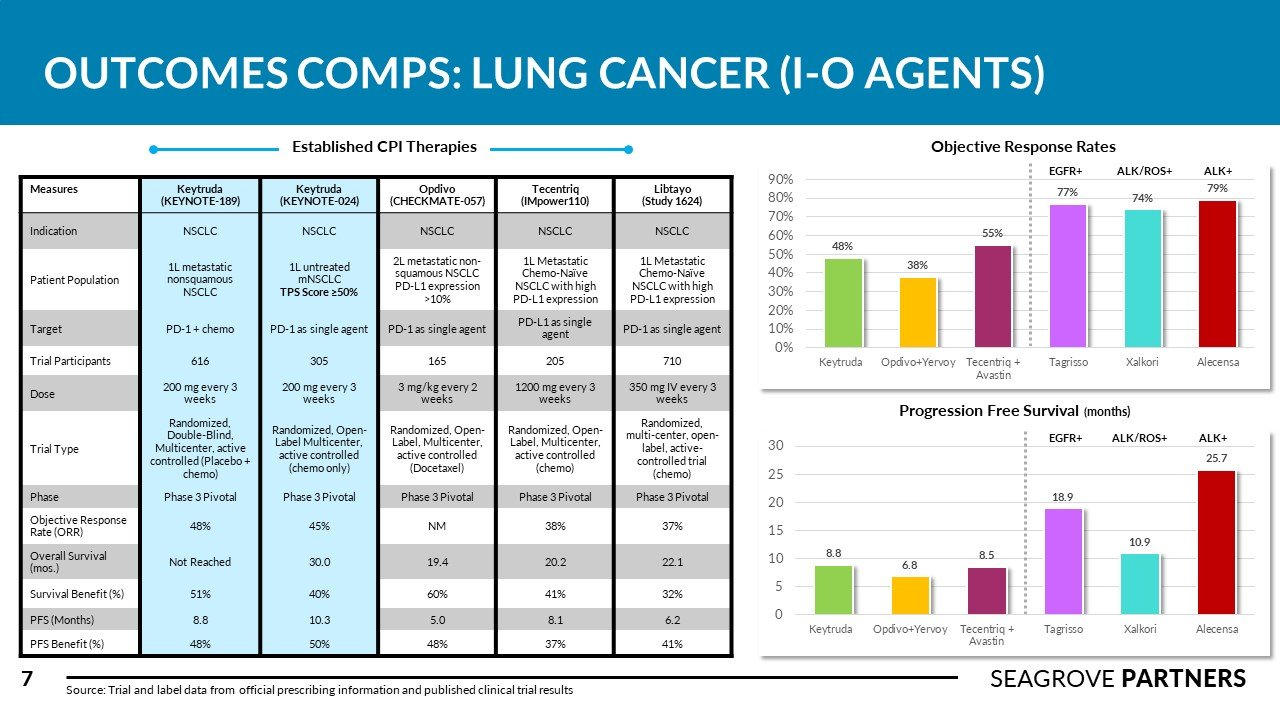
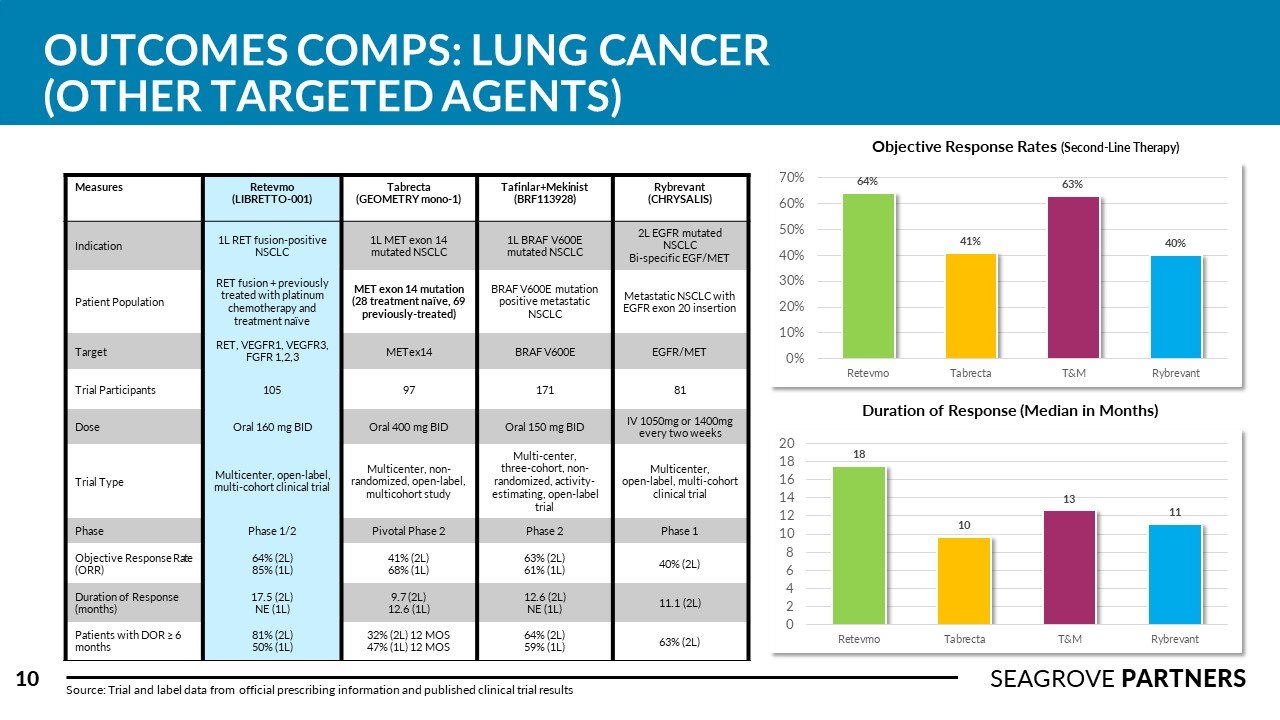
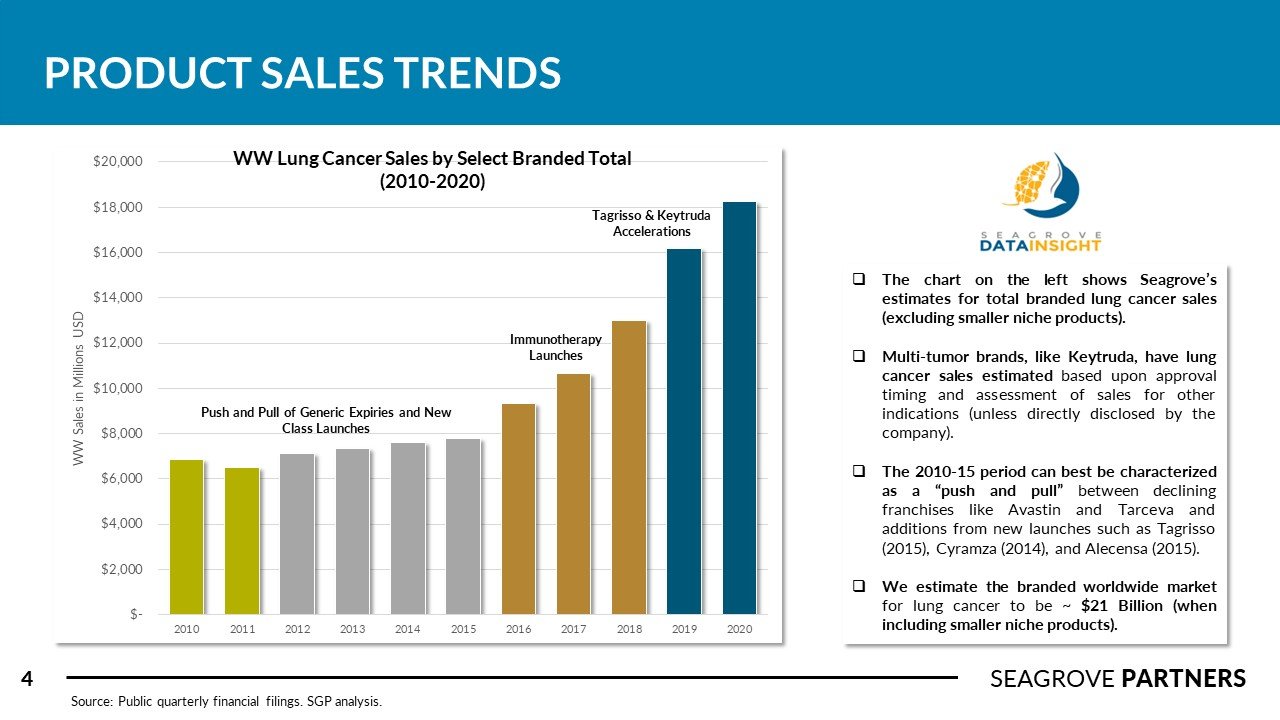
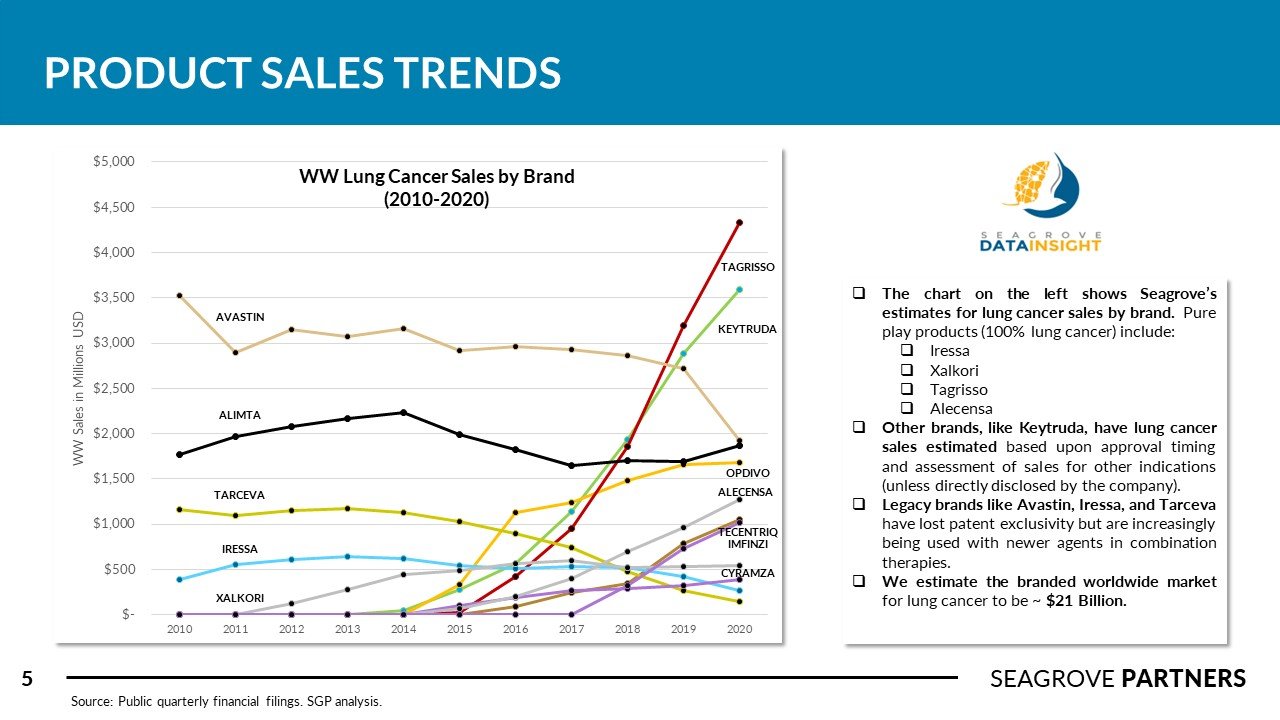
There are over 228,000 new cases of lung cancer in the US every year (NSCLC accounts for about 75% of this total). The overall 5-year survival rate is 21%. Competitors in this space tend to complete numerous clinical studies across different subsets of NSCLC treatment (squamous vs. non-squamous, 1L mono, 1L combined with chemo, 2L in high PD-1/PD-L1 expressed patients). Tagrisso was recently approved for adjuvant use in Stage 1B-3A patients with an 80% improvement is disease free survival (versus placebo). Imfinzi is the only I-O agent approved for use in Stage 3 NSCLC showing 62% survival rate and PFS of 16.8 months (versus 5.6 for placebo). Libtayo, Lumakras, and Rybrevant were approved recently adding important new therapy options for different sub-groups of NSCLC patients. Seagrove Partners estimates the WW lung cancer drug market to be ~ $21 billion.